2025-01-10 DP TechnologyHaiPress
BEIJING,Jan. 9,2025 --In a recent achievement,DP Technology's hit discovery platformRiDYMO® has successfully designed a cyclic peptide targeting β-catenin,a historically "undruggable" protein,in just two months. This innovative AI-driven approach combines advanced algorithms,physics simulations,and high-throughput experimentation to rapidly develop potential drug candidates for challenging targets. The platform's success with β-catenin,a key player in cancer development,demonstrates its potential to revolutionize drug discovery for previously intractable targets.
The Wnt/β-catenin pathway plays a crucial role in various biological functions and is implicated in multiple cancers[1-3]. Despite its importance as a therapeutic target[4-7],β-catenin has long been considered "undruggable" due to its flat protein surface[8-10]. The RiDYMO® platform overcomes this challenge by leveraging a vast library of over 10^12 cyclic peptides and macrocyclic molecules,incorporating both natural and non-natural amino acids.
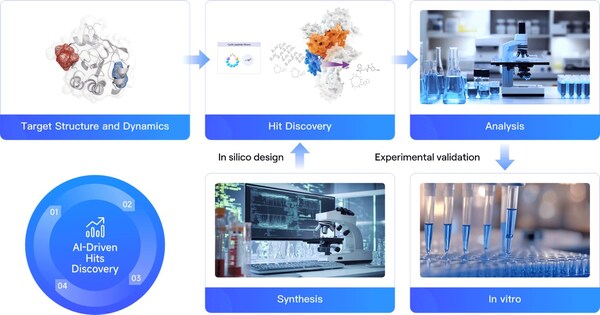
Fig. 1 RiDYMO® : an AI for Science-Driven Hit Discovery and Optimizaton Platform
Using the RiDYMO® platform,researchers rapidly designed and screened cyclic peptide molecules for β-catenin binding. The process involved:
Optimizing the β-catenin protein structure to improve the binding interface.
High-throughput screening and design of cyclic peptides using the platform's extensive library.
Synthesis and testing of 29 candidate molecules.
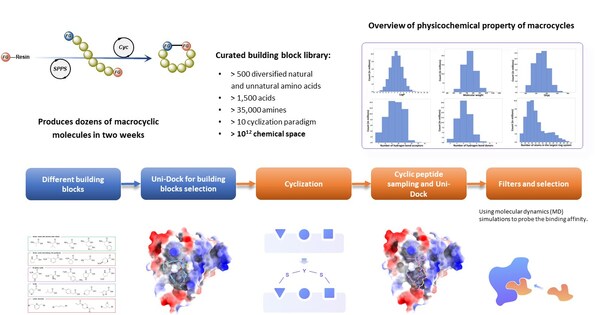
Fig. 2 The workflow of the Cyclic Peptide Screening Process Based on the RiDYMO® Platform
The results were impressive,with 12 compounds showing strong inhibition of protein-protein interactions (IC50≤10 μM),a success rate of over 40%. One standout compound,designated as compound 24,effectively bound to β-catenin,disrupted its interaction with BCL9,and inhibited the Wnt/β-catenin signaling pathway.
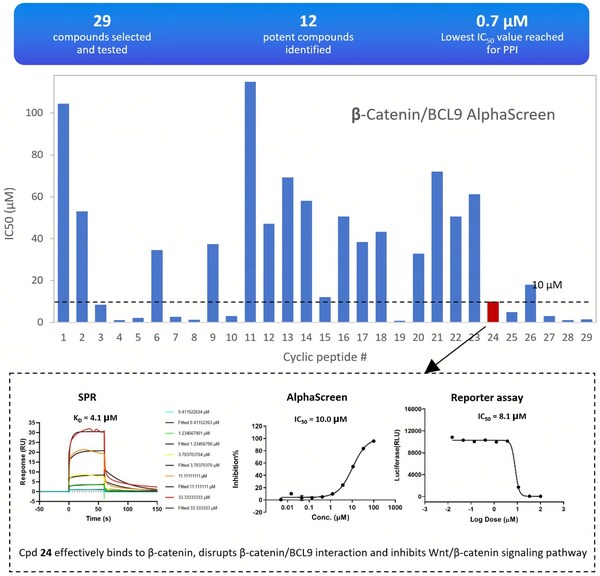
Fig. 3 Experimental Results of 29 Cyclic Peptide Molecules
Dr. Dongdong Wang,Co-President of DP Technology's Drug Discovery Unit,emphasized the platform's ability to tackle undruggable targets and rapidly design high-quality compounds. The RiDYMO® platform's success with β-catenin demonstrates its potential to accelerate the development of orally available cyclic peptide drugs,potentially transforming the pharmaceutical industry's approach to challenging targets.
This breakthrough in cyclic peptide design for β-catenin showcases the power of AI-driven drug discovery platforms in addressing previously intractable targets,potentially opening new avenues for cancer treatment and other therapeutic areas.
About the RiDYMO® Hit Discovery and Optimization Platform
RiDYMO® is a state-of-the-art hit discovery and optimization platform developed by DP Technology,leveraging the principles of AI for Science. It employs the proprietary Hermite® computational drug design software to elucidate the dynamics of "undruggable" targets and explores a broader chemical space encompassing small molecules,macrocycles,and cyclic peptides. By integrating advanced artificial intelligence,physics-based algorithms,and high-throughput experimentation,the platform excels in designing oral macrocyclic compounds and rapidly delivers innovative drug candidates.
As one of its core algorithms,Reinforced Dynamics (RiD)[4] has a significant advantage in the sampling efficiency of molecular dynamics simulation. By fully leveraging the high-dimensional representation capabilities of neural networks,RiD can efficiently capture dynamic conformational changes in complicated biomolecular systems. Previously,the core RiD algorithm of the platform was published in Nature Computational Science [11]. The study demonstrated that RiD could achieve a more comprehensive free energy surface within 1.86 μs,compared to 100 μs required by traditional MD methods,representing nearly a hundredfold increase in efficiency.
RiDYMO® has been successfully employed in various drug discovery programs,including the development of small molecule compounds for crucial targets such as c-Myc and GPX4,as well as other modalities such as cyclic peptides and ADCs.
For more information,please visit our website.
(https://www.dp.tech/en/services/medicine).
About Hermite
Hermite® is a next-generation computational drug design platform developed by DP Technology,powered by AI for Science,to provide a comprehensive solution for drug design. Hermite® integrates industry-leading tools such as the Free Energy Perturbation module ("Uni-FEP"),and the ultra-high-throughput virtual screening tool ("Uni-VSW"). The platform supports key stages of drug discovery from protein structure prediction and target validation to hit discovery and lead optimization.
In addition,the platform offers an interactive,web-based molecular visualization experience,with detailed management of projects,teams,and data. It features full compliance certification,multi-tier security measures. Users have the flexibility to choose between cloud-based or private deployment options.
Hermite® is rapidly gaining traction in the pharmaceutical industry,with over 60% of leading companies in China utilizing the platform across more than 50 drug pipelines. This growing trust is reflected in the impressive number of calculations performed—over 200,000 Uni-FEP calculations to date—demonstrating Hermite®'s reliability and effectiveness as a powerful tool for drug discovery and development.
For more information,please visit: https://www.dp.tech/en/product/hermite
References:
Salik B,et al. Targeting RSPO3-LGR4 signaling for leukemia stem cell eradication in acute myeloid leukemia. Cancer Cell. 2020;38(2):263–78.e6.
Liu J,et al. Wnt/β-catenin signal ling: function,biological mechanisms,and therapeutic opportunities. Signal Transduct Target Ther. 2022 Jan 3;7(1):3.
Choi BR,Cave C,Na CH,Sockanathan S. GDE2-dependent activation of canonical wnt signaling in neurons regulates oligodendrocyte maturation. Cell Rep. 2020;31(5):107540.
Zhang L,Shay JW. Multiple roles of APC and its therapeutic implications in colorectal cancer. J. Natl. Cancer Inst. 2017;109(8).
Belenguer G,et al. RNF43/ZNRF3 loss predisposes to hepatocellular-carcinoma by impair ing liver regeneration and altering the liver lipid metabolic ground-state. Nat. Commun. 2022;13(1):334.
Cao L,et al. Helicobacter pylori-induced RASAL2 Through Activation of Nuclear Factor-κB Promotes Gastric Tumorigenesis via β-catenin Signaling Axis. Gastroenterology. 2022 May;162(6):1716-1731.e17.
Hashemi M,et al.,Biological functions and molecular interactions of Wnt/β-catenin in breast cancer: Revisiting signaling networks. Int. J. Biol. Macromol. 2023 Mar 31:232:123377.
Hwang et al.,Direct Targeting of b-Catenin by a Small Molecule Stimulates Proteasomal Degradation and suppresses Oncogenic Wnt/β-Catenin Signaling. 2016,Cell Rep. 16,28–36.
Tanton et al.,A novel β-catenin/BCL9 complex inhibitor blocks oncogenic Wnt signaling and disrupts cholesterol homeostasis in colorectal cancer. 2022,Sci. Adv.8,eabm3108.
Scuoppo C. et al.,"ST316,a Clinical Peptide Antagonist of β-catenin,Induces Anti-Tumor Immune Responses by Multiple Mechanisms of Action." AACR 2024.
Wang D,et al. Efficient sampling of high-dimensional free energy landscapes using adaptive reinforced dynamics. 2022,Nat. Comp. Sci. 2,20.
02-22
02-19
02-19
02-16
02-13
02-13
02-12
02-12
02-12
02-11